Calculate the Wavelength of a Photon
The formula used to calculate the wavelength of an electromagnetic wave is. The Wavelength of all spectral lines is the wavelength of all the spectral or energy lines or levels in all the series is calculated using Wavelength of photon Initial orbit 2Final orbit 2R Atomic Number 2Final orbit 2-Initial orbit 2To calculate Wavelength of all spectral lines you need Initial orbit n i Final orbit n f Atomic Number Z.
Lambda dfrac hc E_ ph λ E p.

. Enter the energy per photon for the electromagnetic wave. Ehfhcλ λhcE Where h Plancks constant 661034 Js E Energy in joules c speed of light 3108 ms The photon is a type of elementary particle it has no mass and travels with speed of light. Here c is the speed of the light E is the photon energy and h is the Plancks constant.
Substituting the values we get λ 662 10 34 3 10 8 1602 10 19. E hc l equation 1 where h is Plancks constant 66261 x 10 -34 Js and c is the speed of light 29979 x 10 8 ms. The formula for calculating wavelength is.
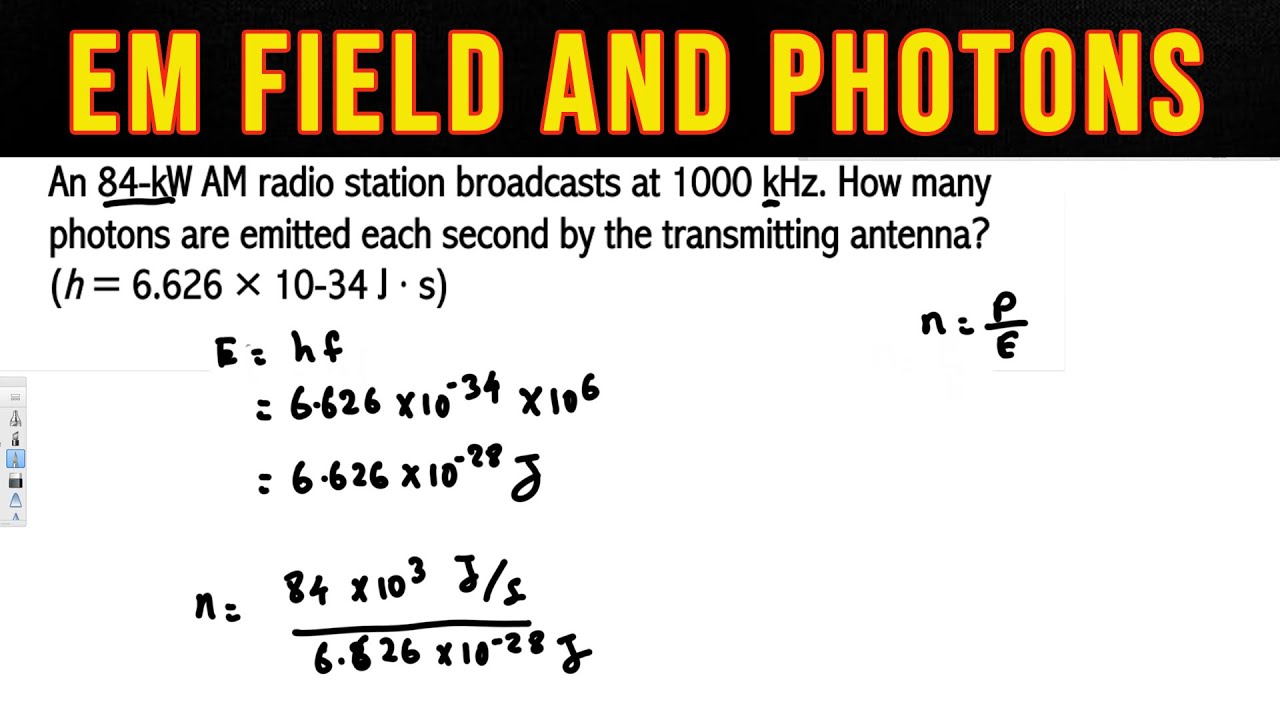
To find the wavelength of a wave you just have to divide the waves speed by its frequency. An Energy of a Photon Calculator. We will calculate the energy using the equation for the wavelength of a photon.
Wavelength can be calculated using the following formula. So the wavelength of photon having energy 1 e V is 12397 10 3 A. In other words the energy of a photo is directly proportional to its frequency and inversely proportional to its wavelength.
W a v e l e n g t h W a v e s p e e d F r e q u e n c y displaystyle Wavelengthfrac WavespeedFrequency. When an electron changes from one atomic orbital to another the electrons energy changes. E h c λ h f E photons energy H Planck constant C lights speed λ photons wavelength F photons frequency Light is a collection of particles and this formula gives us the single indivisible quanta of light.
From equations 1 and 2 we can write. Wavelength usually is expressed in units of meters. Photon wavelength calculator - WolframAlpha.
λ 12397 10 7 m. λ h c E p h. Calculate the wavelength in nm of a photon emit- ted by a hydrogen atom when its electron drops from the n 5 state to the n 3 state.
4 Because the speed of light c is constant and has a value of 3 X 10 8 ms we may deduce from the above equation that the wavelength. 3 8 1 0 1 8 J. But 1 A 1 10 10 m.
You can also select the units if any for Input s and the Output as. λ h c E. E p h ν h c λ h c κ.
What is the wavelength of the photon formula. The Rydberg formula is a mathematical formula used to predict the wavelength of light resulting from an electron moving between energy levels of an atom. λ 1986 10 26 1602 10 19.
6 2 6 1 0 3 4 J s. The wavelength of the photon can be calculated by. Photon Energy Joule Output.
The equation for Planck looks like this. The symbol for wavelength is the Greek lambda λ so λ vf. All that remains is to plug in the values and get the answer.
Hν λhc. Efrachclambda Efrac414times 10-15times 3times 108725times 10-9 E17 mathrm. E_ p hnu dfrac hc lambda hckappa E p.
H approx 6626cdot 10. The energy of light or photon energy E is inversely proportional to the wavelength by the equation. Calculate the wavelength in nm of a photon emit- ted by a hydrogen atom when its electron drops from the n.
Example Photon Energy calculator1. When the electron changes from an orbital with high energy to a lower energy state a photon of. Light wavelength 05 µm.
Volume of a cylinder. To calculate Energy of photon you need Wavelength of photon λ. Following calculator calculates photon energy based on light wavelength.
E hν. The wavelength of the photon formula is λ h x cE. C 0 Speed of light in a vacuum.
Its energy is given by E hf and is related to the frequency and wavelength of the radiation by E h f λ h c where. Wavelength wave velocityfrequency. λ Wavelength of EM wave.
λ h c 0 E. Therefore λ 12397 10 7 1 10 10. λ 12397 10 3 A.
E Photon energy. E 6626 x 10 -34 Js x 3 x 10 8 msec 633 nm x 10 -9 m1 nm. Photon Energy and Wavelength.
Use Math Input Mode to. 3 Thus the wavelength of the photon is given by. Calculate wavelength with the wavelength equation.
H Planck constant. 1 Calculate the energy of one photon of yellow light with a wavelength of 589 nm A photon is a quantum of electromagnetic radiation and each photon possesses some energy depending on the frequency of the radiation. 6 2 6 1 0 3 4.
Energy of photon light is calculated using Energy of photon hP c Wavelength of photon. Photon Energy 397566 e-19 Joule. With our tool you need to enter the respective value for Wavelength of photon and hit the calculate button.
And the photon wavelength is. Light wavelength µm Input. What is the wavelength A of a photon that has an energy of 4.

What You Need To Know About The Rydberg Formula And How To Use It Formula Mathematical Equations Need To Know

De Broglie Relation Hypothesis Free Energy Generator Relatable

Pin By Gan On The Tear By Ian Mcdonald Planck Constant Lesson Electromagnetic Radiation
No comments for "Calculate the Wavelength of a Photon"
Post a Comment